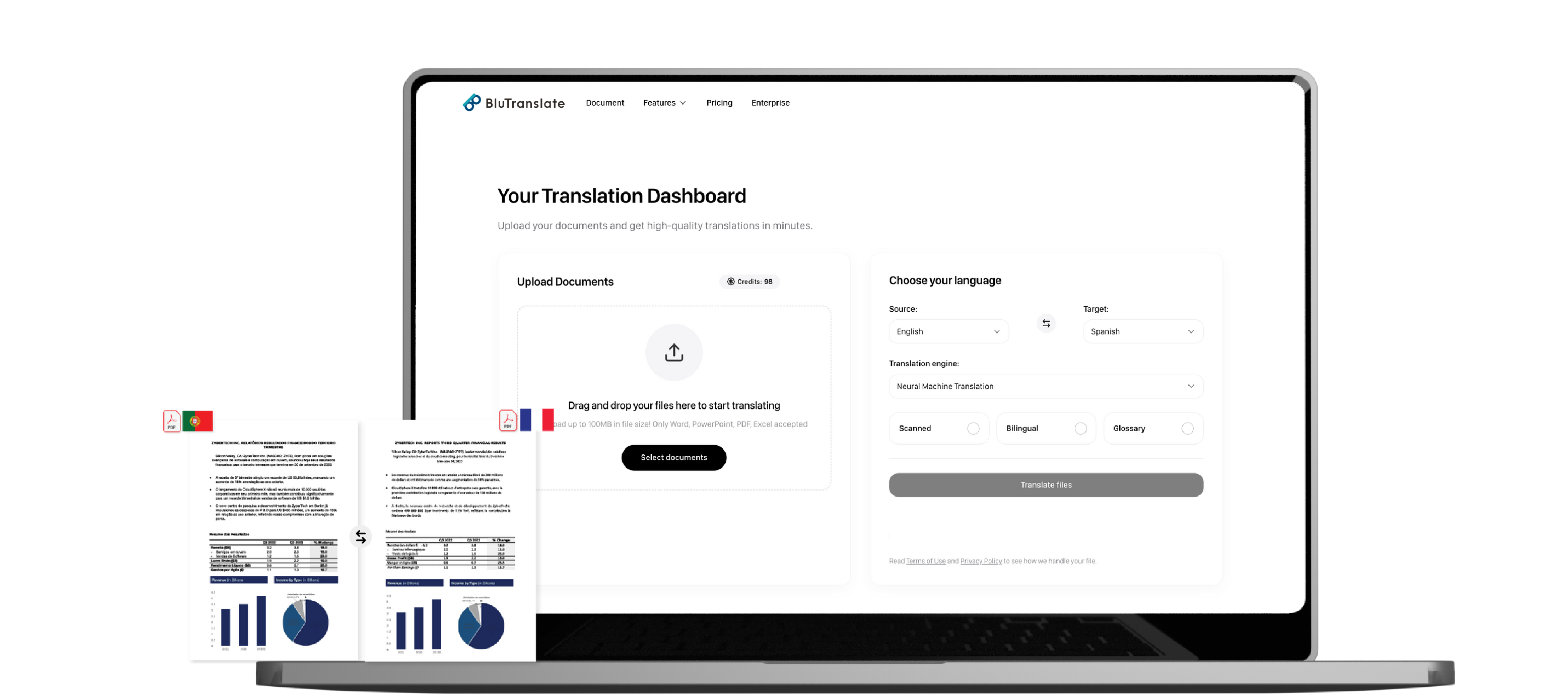
Submission-Ready Pharmaceutical Labeling Translation
Avoid costly submission delays. Bluente's AI platform provides secure pharmaceutical labeling translation that keeps your formatting perfectly intact.
Need certified translation for official submissions?
Trusted by employees of















Format-Perfect Pharmaceutical Label Translations
Pharmaceutical labeling requires absolute precision and formatting accuracy for regulatory approval. Traditional translation services often disrupt critical formatting elements, leading to delays, compliance issues, and increased costs.
Bluente's AI platform specifically addresses these challenges by preserving complex structures like tables, charts, and legal numbering across all pharmaceutical documentation. Our technology ensures that multilingual labeling maintains data integrity while delivering the speed necessary for competitive global submissions.
For regulatory affairs teams facing tight deadlines, our layout-aware translation engine allows you to translate pharmaceutical labels, package inserts, and compliance documentation without sacrificing format or accuracy. The bilingual outputs facilitate efficient review processes, accelerating your path to multi-market compliance.
What's included in our instant document translation?
Bluente gives you fast, secure, and scalable access to high-quality translations across 120+ languages for your documents.
Our advanced terminology recognition and layout preservation deliver exceptional accuracy, trusted by legal and finance professionals who require precision.
Instant Document Translation
Fast, reliable document translation for seamless workflows.
- 120+ languages
Comprehensive language coverage for global reach
- Multi-format support: PDF, DOCX, PPTX, XLSX, images
Translate all your document types
- Layout-aware engine
Preserves tables, charts, footnotes, numbering, and styles
- Advanced OCR for scanned documents
Convert non-selectable text into editable, searchable content
- Bilingual and review-ready outputs
Side-by-side originals/translations with tracked changes
- Enterprise-grade security
End-to-end encryption and automatic file deletion
- Fast, scalable processing
Large files and batches processed within minutes
- Format-perfect translation
Original layout, styling, and structure maintained
How it works
Our streamlined process delivers fast, accurate, and format-perfect document translations.
Upload Your Files
Securely upload your documents (PDF, Word, Excel, images, etc.) to our platform.
Select Languages & Settings
Choose your target languages and any specific translation or formatting preferences.
Receive Instant Translation
Our AI engine processes your documents, preserving layout and delivering high-quality translations in minutes.
Review & Download
Download your translated documents, ready for immediate use, with original and translated content side-by-side.
Frequently Asked Questions
Get answers to common questions about our online document translation service. Learn about supported formats, security, turnaround time, and how to get started with instant translations.
Bluente's layout-aware engine preserves all critical formatting elements including tables, charts, footnotes, legal numbering, and specialized styling across pharmaceutical labeling documents. Our AI platform is specifically designed to maintain the exact placement of images, regulatory symbols, and complex numbering systems required for pharmaceutical submissions. This ensures your translated labels remain submission-ready without manual reformatting.
Bluente supports multiple document formats commonly used in pharmaceutical labeling, including PDF, DOCX, PPTX, XLSX, images (JPG/PNG/TIFF), XML, JSON, TXT, and CSV. Our platform also features advanced OCR capabilities that can convert non-selectable text in scanned documents into editable, searchable, and translatable content while maintaining the original layout and formatting.
Bluente implements enterprise-grade security measures including encryption and automatic file deletion to protect sensitive pharmaceutical information. Our platform is designed with confidentiality in mind, ensuring that proprietary formulations, clinical data, and regulatory submissions remain secure throughout the translation process.
Bluente's AI platform translates pharmaceutical labeling documents within minutes rather than days, dramatically reducing turnaround time. Our system efficiently handles large files and multi-document batches, enabling time-sensitive regulatory submissions across multiple markets simultaneously.
Bluente generates bilingual and review-ready outputs that place original and translated content side by side, facilitating thorough regulatory review and verification. This capability is especially valuable for pharmaceutical compliance teams who must ensure exact correlation between source and translated content across all regulatory documentation.
Yes, Bluente's advanced OCR technology can translate scanned pharmaceutical package inserts, labels, and other documentation. The platform converts non-selectable text in scanned PDFs or images into editable, searchable, and translatable content while preserving the original layout, making it ideal for legacy documentation or third-party materials.
Translate Pharmaceutical Labels Now
Secure, regulatory-ready translations with perfect formatting. Get submission-ready document translations in minutes across 120+ languages.
Need certified translation for court or immigration?
Get Certified Translation →