Clinical Research Translation for Compliant Global Trials
Ensure ethical approval and legal integrity for patient consent forms. Bluente's AI preserves exact formatting and security for compliant translations.
Need certified translation for official submissions?
Trusted by employees of















Why Clinical Research Translation Demands Precision
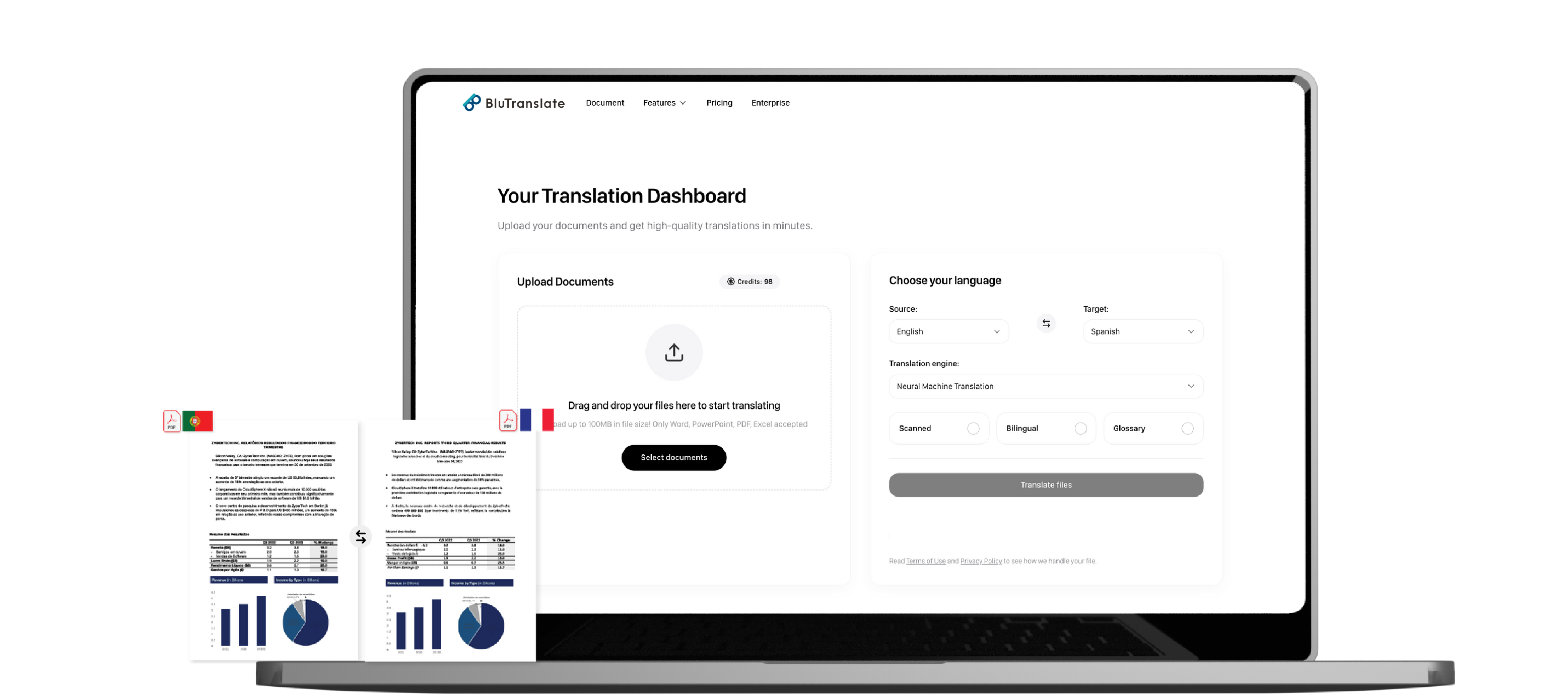
Clinical research documentation requires meticulous translation that preserves both meaning and structure. Generic translation tools often break critical layouts, scramble tables of trial data, and fail to maintain the numbering systems essential for regulatory submissions.
Bluente's AI document translation platform was built specifically to solve these challenges. Our technology preserves tables, charts, legal numbering, and complex formatting across all your clinical trial documents—from protocols and consent forms to statistical analyses.
With enterprise-grade security, our platform protects sensitive patient data through encryption and automatic file deletion. Generate bilingual, side-by-side outputs for efficient review processes, accelerating cross-border collaboration while ensuring complete data integrity.
Translate large batches of research documents in minutes instead of days, maintaining compliance standards without sacrificing speed or accuracy.
What's included in our instant document translation?
Bluente gives you fast, secure, and scalable access to high-quality translations across 120+ languages for your documents.
Our advanced terminology recognition and layout preservation deliver exceptional accuracy, trusted by legal and finance professionals who require precision.
Instant Document Translation
Fast, reliable document translation for seamless workflows.
- 120+ languages
Comprehensive language coverage for global reach
- Multi-format support: PDF, DOCX, PPTX, XLSX, images
Translate all your document types
- Layout-aware engine
Preserves tables, charts, footnotes, numbering, and styles
- Advanced OCR for scanned documents
Convert non-selectable text into editable, searchable content
- Bilingual and review-ready outputs
Side-by-side originals/translations with tracked changes
- Enterprise-grade security
End-to-end encryption and automatic file deletion
- Fast, scalable processing
Large files and batches processed within minutes
- Format-perfect translation
Original layout, styling, and structure maintained
How it works
Our streamlined process delivers fast, accurate, and format-perfect document translations.
Upload Your Files
Securely upload your documents (PDF, Word, Excel, images, etc.) to our platform.
Select Languages & Settings
Choose your target languages and any specific translation or formatting preferences.
Receive Instant Translation
Our AI engine processes your documents, preserving layout and delivering high-quality translations in minutes.
Review & Download
Download your translated documents, ready for immediate use, with original and translated content side-by-side.
Frequently Asked Questions
Get answers to common questions about our online document translation service. Learn about supported formats, security, turnaround time, and how to get started with instant translations.
Bluente's AI-powered platform combines linguistic accuracy with layout preservation specifically designed for complex documents. Our system maintains the integrity of tables, charts, footnotes, and legal numbering—critical elements in clinical research documentation. The platform produces bilingual, side-by-side outputs that allow for efficient comparative review, ensuring terminology consistency and structural accuracy across all translated materials.
Bluente supports a comprehensive range of formats commonly used in clinical research, including PDF, DOCX, PPTX, XLSX, images (JPG/PNG/TIFF), XML, JSON, TXT, and CSV. Our advanced OCR capability also processes scanned documents, converting non-selectable text into editable, searchable, and translatable content—essential for older trial documentation or regulatory submissions received as scanned copies.
Patient consent forms require precise formatting to maintain ethical and legal compliance. Bluente's format-perfect translation technology preserves the exact layout, styling, tables, and legal numbering of consent documents. This ensures that translated versions maintain the same structural integrity as originals, which is essential for ethical committee approvals and regulatory compliance across multiple trial sites and jurisdictions.
Bluente implements enterprise-grade security protocols including end-to-end encryption during processing and automatic file deletion after translation. This ensures patient data and proprietary research information remain confidential and compliant with data protection regulations. Our secure infrastructure is designed specifically for handling sensitive materials like clinical protocols, patient information, and trial results.
Bluente's platform translates large files and multi-document batches within minutes, not days. This rapid processing capability is particularly valuable for time-sensitive clinical research workflows, such as preparing documentation for regulatory submissions, translating amendments for ethics committees, or managing cross-border trial communications. The speed doesn't compromise quality—all translations maintain format perfection and accuracy.
Yes, Bluente's advanced OCR technology efficiently handles scanned historical trial data and protocols. The system converts non-selectable text in scanned PDFs or images into editable, searchable, and translatable content while preserving the original formatting. This capability is invaluable for accessing and translating legacy research documentation, older regulatory submissions, or paper-based trial records that need to be included in current research activities.
Translate Your Clinical Research Documents
Format-perfect translations with regulatory compliance in mind. Secure, accurate, and ready in minutes—not days.
Need certified translation for court or immigration?
Get Certified Translation →